Interventional Care

We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
What is the contact time for Sani-24® Germicidal Spray and Wipe?
The Sani-24 Wipe is effective against 27 microorganisms, including SARS-CoV-2, within a 1-minute contact time. Additional organisms are included within a 5-minute contact time; specific details listed below:
Figure 1. List of Disinfection Organisms*

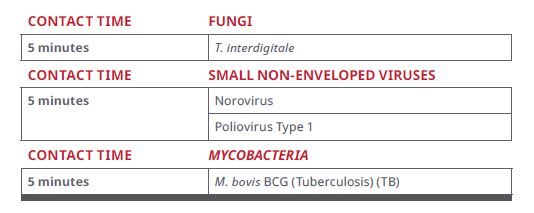
Figure 2. List of Residual Disinfection Organisms (Continuously Active Disinfection)*
Upon following the directions for use for Continuously Active Disinfection, disinfection occurs within 5 minutes for certain organisms listed below following contamination events or touches for up to 24 hours post application.
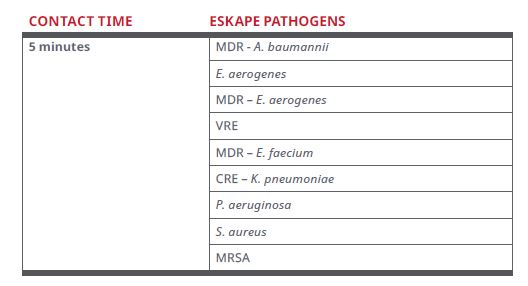
*The above organisms names may be abbreviated. Please refer to the Sani-24 Technical Data Bulletin for detailed list of organism reference numbers and strain information.
What is the shelf life for Sani-24® Germicidal Spray and Wipe?
The shelf life for the Sani-24® Wipe and Spray is 24 months or 2 years from the date of manufacture. Product can be used until the expiration date, even after being opened. Expiration date is considered the last day of the month of expiry.
Are eye wash stations required for areas where Germicidal Disposable Wipes and Germicidal Sprays are utilized?
Eye wash stations are not required for areas where Germicidal Disposable Wipes and Germicidal Sprays are utilized as intended for surface disinfection. Although these products are classified as eye irritants, there is no OSHA or ANSI regulation that requires an eye wash station for eye irritants and therefore one not needed for these products. Handling of any chemical product, whether category 1, 2, 3, or 4, include using PPE when required, engineering controls if appropriate, and safe work practice controls to minimize any risk of exposure
Is PDI, Inc. a publicly traded company?
No. PDI is a privately held, third generation, family-owned company and is therefore, not publicly traded. For more information on the history of PDI, please visit the “About” section of the website.
What year was PDI established?
Professional Disposables International, Inc., commonly known as PDI, Inc., was founded in 1977 as the healthcare division of Nice-Pak Products, Inc. and, in 2007, was established as a separate entity. For more information on the history of PDI, please visit the “About” section of the website.
Does PDI offer continuing education programs for medical professionals?
Yes. PDI’s Clinical Science Liaisons can deliver a variety of continuing education programs onsite and/or via conferencing platforms. To learn more about offerings, please contact your local Clinical Science Liaison via customer care at 1-800-999-6423.
What is the relationship between PDI, Inc. and Nice-Pak Products, Inc.?
PDI is an affiliate of Nice-Pak Products, Inc., global leader in wet wipe products. PDI Healthcare is a division of PDI, Inc., which also includes PDI Contract Manufacturing and Sani-Professional businesses. For more information, visit www.wearepdi.com
For more information about Nice-Pak, visit www.nice-pak.com.
For more information about Contract Manufacturing, visit www.pdicontract.com/drupal7/
For more information about Sani-Professional, visit www.saniprofessional.com
What is the expiration date on Sani-Hands® Instant Hand Sanitizing Wipes?
Sani-Hands wipes have a 24 month expiration date from the date of finished product manufacturing.
Are Sani-Hands® Instant Hand Sanitizing Wipes an FDA regulated product?
Sani-Hands Instant Hand Sanitizing Wipes are regulated by the US FDA as an OTC (Over-the-Counter) drug product covered under the Tentative Final Monograph for Healthcare Antiseptic Drug Products.
When is the Profend™ Nasal Decolonization Kit used?
The Profend Nasal Decolonization Kit can be used on patients who have tested positive for S. aureus and/or MRSA. As an alternative to a “test and treat” strategy, the ease and speed of application and economical design make Profend suitable for universal decolonization of all patients, saving time and money spent on patient testing. Regardless of decolonization strategy, Profend has proven efficacy—in a study of healthy volunteers, the Profend Nasal Decolonization Kit reduced S. aureus by 99.7% at 1 hour and 99.9%* at 12 hours post-application.With this in mind, for pre-operative use, clinicians may choose to apply the product to the patient 1-2 hours prior to surgery depending on hospital protocol.
SOURCE: PDI in vivo Study 0113-CTEVO.
Who uses the Profend® Nasal Decolonization Kit?
Perioperative nurses who care for patients prior to surgery and nursing staff in other areas of the hospital,ie. ICU, are the primary users of the Profend Nasal Decolonization Kit. Profend offers unique features valued by clinicians – a preference study showed that over 90% of nurses preferred Profend over other PVP-I nasal antiseptic products.
SOURCE: PDI user acceptance study.
Where is the Profend™ Nasal Decolonization Kit used?
The Profend Nasal Decolonization Kit can be used anywhere in a healthcare facility where there are patients who may be nasally colonized with S. aureus or MRSA and therefore have an elevated risk of developing an SSI or other HAIs.