Interventional Care

We notice that you are visiting us from . This site only services US-based visitors. Would you like to visit the site that is appropriate for your location?
Does NFPA 30 (National Fire Protection Association’s Flammable and Combustible Liquids Code) apply to the Sani-24 Wipe?
NFPA 30 applies to the storage of flammable and combustible liquids. The Sani-24 Wipe is exempt from the requirements of NFPA 30 because the scope of NFPA 30 (Section 1.1) states that it applies to the storage, handling, and use of flammable and combustible liquids. Even though our products contain liquids that have been classified as flammable on the SDS, the wipes themselves are not classified as liquid products. Rather, they are wipes impregnated with flammable liquids, even though there may be a small amount of free liquid in the bottom of the canister. According to the EPA, wipes are classified as a mixture product containing solids and liquids. The NFPA has also confirmed that wipes are not considered to be liquid for the purposes of NFPA 30.
How does the lid on the Sani-24 Wipe lid help make disinfection easier and more efficient?
The Sani-24 Wipe features PDI’s new Dual Access Lid, an intuitive design that enables quick dispensing of a single wipe or multiple wipes. There is a wide opening for rapid initial thread and reload, as well as a flip cap featuring Snap & Close Technology and definitive open/close positions to help eliminate wipes drying out.
What is the compatibility profile on the Sani-24 Wipe?
The Sani-24 Wipe is compatible with a broad range of surfaces and materials found in healthcare, including ABS plastic, stainless steel, and porcelain/marble.
Is the Sani-24 Wipe intended to help a facility consolidate SKUs?
This will be a facility-specific choice. The Sani-24 Wipe may replace other quat/alcohol disinfectants currently in use to standardize SKUs. Or Sani-24 Wipe’s CAD technology can be used to supplement the current disinfectant products within the facility as part of a layered strategy for surface and equipment disinfection.
Where are some ideal areas the Sani-24 Wipe can be used in the healthcare setting?
The Sani-24 Wipe may be used as a routine general surface disinfectant.
Conversations with IP and EVS may address where CAD technology would benefit the most i.e. Areas to keep in mind by asking the question, “Do you think that surface/piece of equipment is disinfected as often as it needs to be?” The following are examples, not all inclusive, of surfaces/areas that may not be as frequently disinfected as they should:
What key units or departments can be targeted for the clinical benefit of the Sani-24 Wipe?
The Sani-24 Wipe and its CAD technology would be beneficial in any unit or department, including ICUs, NICUs, Oncology, EDs, Surgical Services, Dialysis, Patient Rooms, Waiting Rooms, etc.
Would the Sani-24 Wipe ideally be used by EVS or Nursing or both?
Both, depending on the needs of the facility and the ownership of cleaning of the chosen areas where the Sani-24 Wipe is to be utilized. Some facilities may even have volunteers overseeing the cleaning and disinfection of different pieces of equipment (ex. wheelchairs).
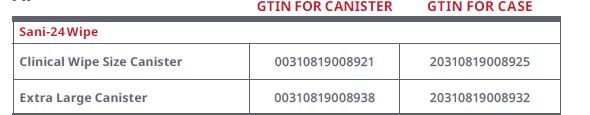
What are the Global Trade Item Numbers (GTINs) for the Sani-24 Wipe?
Does the Sani-24 Wipe have an emerging viral pathogen claim?
Yes, the Sani-24 Wipe meets the emerging viral pathogen claim based on efficacy claims for norovirus and poliovirus. The customer should follow the instructions for the norovirus and poliovirus 5-minute contact time, unless a claim for the emerging pathogen has been specifically tested for and approved (i.e., SARS-CoV-2).
Does the Sani-24 Wipe have a soft surface sanitization claim?
The Sani-24 Wipe does not have a soft surface sanitization claim. It does have a hard nonporous, non-food contact sanitization claims for E. aerogenes and S. aureus at 10 second contact time.
What is the EPA Registration number for the Sani-24 Wipe?
The EPA Registration Number is as follows:
How does the Sani-24 Wipe differ from standard disinfectants?
After application with standard disinfectants, pathogens can immediately contaminate a surface as soon as they encounter it. The Sani-24 Wipe is the first and only EPA-registered intermediate level disinfectant that provides Continuously Active Disinfection (CAD), breaking the chain of surface re-contamination for 24 hours.